Author Affiliations
Abstract
Introduction: Dexmedetomidine is a highly selective α2-adrenergic receptor agonist with sedative, analgesic, anxiolytic, and anti-shivering properties. Its opioid-sparing effect, minimal respiratory depression, and potential neuroprotective benefits have led to its increasing use across perioperative and critical care settings. This review aims to evaluate recent evidence on the analgesic efficacy, safety, and clinical applications of dexmedetomidine.
Methodology: A systematic literature search was performed using PubMed and Google Scholar databases in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines. Studies published in English between January 1, 2015, and June 30, 2025, involving human subjects and reporting clinical results were included. Case reports, reviews, animal studies, and non-English publications were excluded.
Results: Dexmedetomidine was associated with significant reductions in postoperative pain scores, opioid consumption, and improved analgesic duration across various surgeries, including esophageal endoscopic submucosal dissection (ESD), transurethral resection of the prostate (TURP), shoulder arthroscopy, and total knee arthroplasty (TKA). Pediatric and elderly populations tolerated it well, with minimal respiratory compromise. It prolonged the sensory block duration in spinal anesthesia and enhanced regional anesthesia outcomes. Notably, it improved patient recovery by reducing nausea, agitation, and intensive care unit (ICU) stay. However, dose-dependent bradycardia and hypotension were recurrent findings, emphasizing the need for hemodynamic monitoring.
Conclusion: Dexmedetomidine offers effective, opioid-sparing analgesia and improved perioperative recovery across diverse patient populations. Its safety profile is favorable when dosing is optimized, and cardiovascular parameters are closely monitored. While evidence supports its short-term perioperative benefits, further research is warranted to explore standardized dosing protocols, long-term outcomes, and its potential role in chronic pain management.
Keywords
Dexmedetomidine, Perioperative analgesia, Postoperative pain, Sedation, Critical care sedation.
Introduction
Dexmedetomidine is a potent and highly selective α2-adrenergic receptor agonist. It has drawn considerable interest in recent years because of its versatile applications in clinical settings, specifically in the management of pain. It was first approved by the U.S. Food and Drug Administration (FDA) in 1999 for use as an intravenous sedative in mechanically ventilated patients in the ICU for durations of up to 24 hours.[1] Since its initial approval, dexmedetomidine has been increasingly used for a broad spectrum of anesthetic and analgesic purposes. Distinct from conventional sedatives, it provides sedation, anxiolysis, anti-shivering, and analgesic benefits while preserving respiratory function and causing minimal respiratory depression.[2-4]
Dexmedetomidine achieves its analgesic effects mainly through the activation of central α2-adrenergic receptors, which results in decreased norepinephrine release and altered transmission of pain signals within the central nervous system.[5] The ability to reduce opioid requirements is particularly beneficial in perioperative care as it lowers the need for opioids and thereby minimizes their common side effects, including respiratory depression and gastrointestinal disturbances.[6]
In addition, dexmedetomidine exhibits strong anti-inflammatory and stress-reducing effects. Meta-analyses have shown that it can significantly decrease levels of inflammatory cytokines; however, these effects may vary depending on the clinical context. For instance, in patients undergoing laparoscopic hysterectomy, an intraoperative infusion at a rate of 0.4 μg/kg/h enhanced pain control but had a limited impact on systemic inflammatory markers.[7,8]
Dexmedetomidine has also played a crucial role in minimizing agitation and preventing non-invasive ventilation failure in critically ill patients. Unlike many traditional sedatives, it achieves these benefits without impairing respiratory function, offering a significant clinical advantage in respiratory care.[9] The integration of dexmedetomidine into enhanced recovery after surgery (ERAS) protocols highlights its role in promoting postoperative recovery, primarily through its opioid-sparing effects and enhancement of analgesic outcomes.[10]
Beyond its central sedative and analgesic actions, dexmedetomidine has demonstrated potential in the management of neuropathic pain. It also exhibits neuroprotective effects, which are thought to arise from its antioxidant, anti-apoptotic, and anti-inflammatory mechanisms.[11,12] The sedation produced by dexmedetomidine closely resembles natural sleep, contributing to a lower incidence of delirium, reduced duration of ICU stays, and potential protective effects on organs under ischemic and hypoxic conditions.[13,14]
Despite its numerous clinical benefits, dexmedetomidine requires vigilant monitoring, as it can lead to adverse cardiovascular effects such as hypotension and bradycardia.[15] Nevertheless, dexmedetomidine continues to be a highly adaptable and increasingly essential agent in modern approaches to pain control and sedation. Recent research has broadened insights into the safety and efficacy of dexmedetomidine across diverse patient populations. A notable multicenter study involving elderly patients undergoing orthopedic surgery revealed that perioperative administration of dexmedetomidine significantly lowered the risk of postoperative cognitive dysfunction, reinforcing its potential neuroprotective benefits.[16]
Another randomized controlled trial evaluating dexmedetomidine use during cesarean delivery under spinal anesthesia found that intravenous administration improved both analgesia and sedation, while maintaining stable neonatal Apgar scores. These findings support its safety and efficacy in the context of obstetric anesthesia.[17] In pediatric settings, dexmedetomidine has gained prominence as a preferred sedative, owing to its favourable pharmacokinetic profile and minimal impact on respiratory function. It is increasingly employed for procedural sedation in children, particularly during magnetic resonance imaging (MRI) and cardiac catheterization, where maintaining spontaneous respiration is crucial.[18]
Dexmedetomidine’s potential in the treatment of chronic neuropathic pain is currently under active investigation. Early clinical findings indicate that intravenous administration may help relieve symptoms in individuals with refractory diabetic neuropathy, suggesting promising new therapeutic possibilities for managing persistent neuropathic conditions.[19] Recent pharmacoeconomic analyses have demonstrated that integrating dexmedetomidine into anesthesia protocols can lead to a reduction in overall healthcare expenditures, primarily by shortening ICU stays and minimizing opioid-related adverse events and complications.[20]
Methodology
A systematic literature search was conducted using PubMed and Google Scholar databases according to PRISMA guidelines. The search included publications from January 1, 2015, to June 30, 2025, and was limited to studies published in English. The search strategy employed the terms “Dexmedetomidine” OR dexmedetomidine) AND (“Pain Management” OR analgesia) AND (“perioperative”).
Initially, 529 records were retrieved from databases. Of these, 303 records were removed prior to screening for various reasons, including duplication, irrelevance, or failure to meet the inclusion criteria based on title and abstract. This resulted in 226 records proceeding to the screening phase. Out of the 226 records screened, 128 were excluded based on a review of titles and abstracts. The remaining 98 reports were sought for full-text retrieval. However, 71 reports could not be retrieved due to access issues or unavailability, leaving 27 reports for full-text eligibility assessment.
The 27 full-text articles were assessed for eligibility. Each report was evaluated using the SANRA scoring system. Studies scoring below 10 on the SANRA scale were excluded. A total of 22 reports were excluded at this stage due to insufficient SANRA scores. Finally, five studies met all inclusion criteria and were included in the final qualitative synthesis of the review.
Inclusion criteria:
- Research articles encompassing clinical trials, observational studies, or randomized controlled trials
- Studies conducted on human participants
- Publications available in the English language
- Studies involving both male and female subjects
- Articles published within the timeframe of January 1, 2015, to June 30, 2025
Exclusion criteria:
- Non-research content such as books, editorials, commentaries, letters, official documents, and book chapters
- Case reports, case series, and narrative reviews
- Publications in any language other than English
- Studies based on animal models or in vitro (laboratory-based) experiments
- Articles published prior to January 1, 2015, or after June 30, 2025
- Studies that do not include a section reporting results
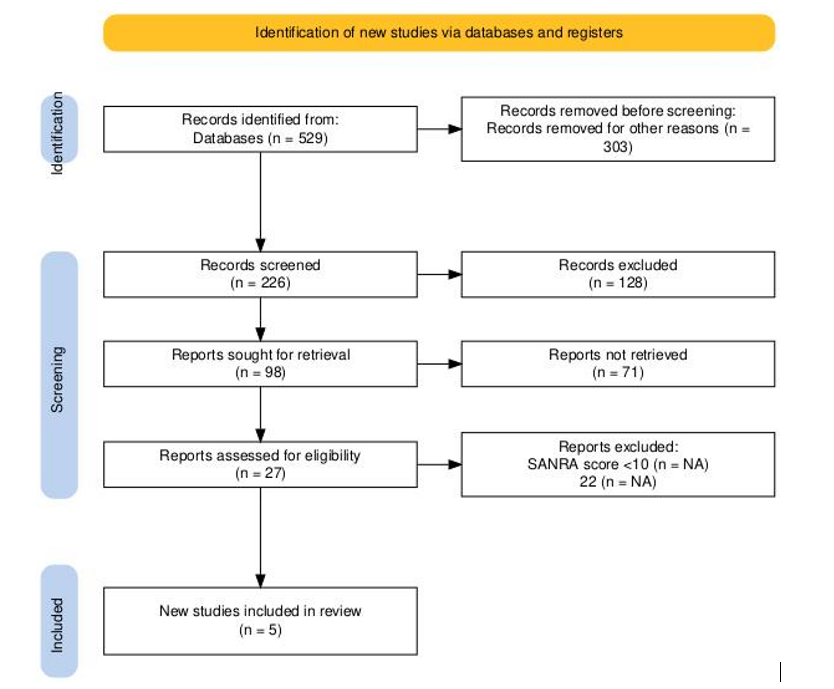
Figure 1: PRISMA diagram
Results
Luo X et al. demonstrated that the addition of dexmedetomidine to anesthesia protocols for esophageal ESD significantly enhanced postoperative outcomes. Patients receiving dexmedetomidine experienced a substantially lower incidence of moderate-to-severe postoperative pain, with an absolute reduction of 33.4% compared to controls (odds ratio (OR): 0.250, 95% confidence interval (CI): 0.085 to 0.731, p = 0.01). One hour after surgery, pain scores were markedly reduced in the dexmedetomidine group (median interquartile range (IQR): 0.5 (2.0) vs 3.0 (1.3), p = 0.003). This analgesic benefit was further reflected by a significant decrease in morphine use in the post-anesthesia care unit (PACU) (0 (0) vs 1.0 (2.0), p = 0.004), indicating a reduced need for rescue opioid analgesia.
In addition to its analgesic effects, dexmedetomidine contributed to a lower incidence and severity of postoperative nausea and vomiting (PONV) and facilitated a shorter PACU stay (p < 0.01), supporting its role in promoting enhanced early recovery. Notably, these benefits were achieved without compromising hemodynamic stability, as the rates of intraoperative hypotension, tachycardia, and bradycardia were comparable between the dexmedetomidine and control groups. Moreover, there were no significant differences in overall hospital stay or patient satisfaction, suggesting that dexmedetomidine use does not adversely affect broader recovery outcomes. These findings underscore the value of dexmedetomidine as an effective adjunct in anesthesia for esophageal ESD, offering robust analgesia, improved recovery parameters, and a favourable safety profile.[21]
The study of Azemati S et al., highlighted the effectiveness of intranasal dexmedetomidine as a premedication for pediatric patients undergoing elective surgery. While ketamine induced faster sedation within the first 30 minutes post-administration, dexmedetomidine exhibited significantly stronger sedative effects at 40 and 50 minutes (p < 0.001 for both), making it particularly suitable for procedures where there is sufficient time for preoperative preparation.
Hemodynamic assessment revealed that dexmedetomidine led to a notable reduction in systolic and diastolic blood pressure, as well as heart rate, in comparison to midazolam and ketamine (p < 0.001). Specifically, systolic blood pressure dropped by 18% in the dexmedetomidine group, while only minimal changes (approximately 3%) were observed in the other groups. Despite these cardiovascular effects, oxygen saturation remained stable, with no instances of hypoxemia (SpO₂ < 95%), affirming the drug’s cardiovascular safety.
In terms of tolerability, dexmedetomidine demonstrated the most favorable side-effect profile. Unlike ketamine, which showed a significantly higher incidence of preoperative nausea and vomiting (p < 0.001), no such effects were reported in the dexmedetomidine group. Postoperative nausea and vomiting were also minimal (6.7%) and did not significantly differ across groups (p = 0.47).
Although not statistically significant, dexmedetomidine provided acceptable conditions for parental separation (73.3% satisfactory) and mask acceptance (40%). Postoperative agitation and pain scores were comparable among all groups, but fewer patients in the dexmedetomidine group required rescue analgesia (16.7%) compared to the midazolam group (33.3%).
In summary, dexmedetomidine proved to be a safe and effective premedication agent in pediatric anesthesia, delivering reliable sedation, stable hemodynamics, and a low side-effect burden when administered 40 to 50 minutes prior to induction.[22]
In a randomized controlled trial, intravenous dexmedetomidine at a dose of 0.4 µg/kg was shown to significantly extend the duration of spinal anesthesia in elderly patients undergoing transurethral resection of the prostate (TURP). The mean time for regression of two dermatomes was notably longer in the dexmedetomidine group (104.44 ± 16.97 minutes) compared to the control group (80.63 ± 15.59 minutes; p < 0.05), indicating a prolonged sensory block. Additionally, patients receiving dexmedetomidine achieved a higher peak sensory block level median T7 (range T6-T8) compared to T10 (range T7-T10) in the control group (p = 0.017), reflecting greater block intensity.
Importantly, this enhancement of sensory anesthesia was not associated with an increased risk of hemodynamic instability, as the incidence of hypotension and bradycardia did not differ significantly between groups. Postoperative pain scores measured at multiple time points (0, 6, 12, and 24 hours) showed no significant differences, though a trend toward lower pain levels was observed in the dexmedetomidine group. Furthermore, the time to the first analgesic request was delayed in the dexmedetomidine group, albeit without reaching statistical significance (p = 0.355).
Multivariable linear regression analysis, adjusting for ASA classification and sensory block height, confirmed that the prolongation of 2-dermatome regression time associated with dexmedetomidine remained statistically significant (p = 0.006). No adverse effects such as hypoxia, excessive sedation, nausea, vomiting, or shivering were reported, underscoring the drug’s safety in this elderly population.
Overall, intravenous dexmedetomidine effectively prolonged and intensified spinal sensory block in TURP procedures, while maintaining cardiovascular stability and a favorable safety profile, supporting its role as a valuable adjunct in spinal anesthesia for elderly surgical patients.[23]
In this randomized, double-blind trial study of Hamed MA et al., the use of dexmedetomidine (0.5 µg/kg) as an adjuvant to bupivacaine in ultrasound-guided erector spinae plane block (ESPB) significantly improved perioperative analgesia in patients undergoing elective shoulder arthroscopy. The addition of dexmedetomidine (ESPB dexmedetomidine group) resulted in a notable reduction in intraoperative fentanyl consumption compared to the bupivacaine-only group (82.86 ± 13.57 µg vs. 100.74 ± 35.07 µg, p = 0.015), suggesting enhanced intraoperative analgesic efficacy.
Postoperatively, the ESPB + dexmedetomidine group exhibited a significantly longer duration before requiring rescue analgesia, with a median time of 18.5 minutes (IQR: 18.25-18.75), compared to 12 minutes (IQR: 12-15.75) in the ESPB-only group (p = 0.044). Additionally, fewer patients in the ESPB + dexmedetomidine group required morphine (p = 0.012), and total 24-hour morphine consumption was significantly lower, with a median of 0 mg (IQR: 0-0) vs. 0 mg (IQR: 0-3) (p = 0.021), highlighting the opioid-sparing benefits of dexmedetomidine.
Overall, the findings affirm that dexmedetomidine, when combined with bupivacaine in ESPB, effectively enhances intraoperative and early postoperative pain control in shoulder arthroscopy. It reduces opioid requirements, prolongs analgesic duration, and maintains a favourable safety profile, with no reported increase in complications.[24]
This randomized controlled study assessed the analgesic effectiveness of intravenous dexmedetomidine combined with femoral nerve block (FNB) in patients undergoing TKA, comparing it to the conventional method of combining FNB with sciatic nerve block (SNB). Patients in the dexmedetomidine group received a preoperative loading dose of 0.6 μg/kg dexmedetomidine intravenously, followed by a continuous infusion of 0.2–0.4 μg/kg/h until surgical closure, alongside an FNB using 0.375% ropivacaine.
Pain scores assessed via the visual analogue scale (VAS) during activity at 24 hours postoperatively, the primary outcome, were not significantly different between the dexmedetomidine and sciatic-femoral (SF) groups. Both groups experienced comparable pain control throughout the perioperative period. The time to first rescue analgesia and the average duration of analgesia (25.4 ± 6.3 hours in the dexmedetomidine group vs. 24.8 ± 6.4 hours in the SF group, p = 0.738) were also similar. Additionally, intraoperative sufentanil usage, maintenance doses of propofol, and rescue analgesic requirements showed no significant differences between the groups (p > 0.05).
Importantly, the safety profiles were comparable, with no increase in adverse event incidence in the dexmedetomidine group. However, a significant prolongation in extubation time was observed in the dexmedetomidine group (p < 0.001), likely due to the sedative properties of dexmedetomidine.
In conclusion, intravenous dexmedetomidine combined with FNB provided postoperative analgesia equivalent to that of the FNB + SNB technique, without increasing adverse effects. This approach may serve as a safer alternative in patients for whom sciatic nerve block poses a risk, while still ensuring effective pain management following TKA.[25]
| Study | Clinical context | Dexmedetomidine dose/route | Key benefits | Opioid/Analgesic reduction | Safety profile | Notable observations |
| Luo X et al.[21] | Esophageal ESD | IV | ↓ Moderate-to-severe postop pain, ↓ PONV, ↓ PACU stay | ↓ Morphine use in PACU | No ↑ hypotension, tachycardia, or bradycardia | 33.4% absolute reduction in pain, improved early recovery |
| Azemati S et al.[22] | Pediatric elective surgery | Intranasal, compared to ketamine/midazolam | Stronger sedation at 40-50 min, ↓ PONV | ↓ Rescue analgesia (16.7% vs 33.3% midazolam) | ↓ BP and HR, stable SpO₂, no hypoxemia | Ideal for premedication with longer prep time |
| Sangkum L et al.[23] | Spinal anesthesia in the elderly | IV 0.4 μg/kg | ↑ Sensory block level (T7 vs T10), longer 2-dermatome regression time | Trend toward delayed first analgesic (NS) | No ↑ in hypotension, bradycardia, or adverse events | Multivariable regression confirmed effect (p = 0.006) |
| Hamed MA et al.[24] | Shoulder arthroscopy with ESPB | ESPB + Dex 0.5 μg/kg | ↓ Intraoperative fentanyl, longer analgesia duration | ↓ 24-hr morphine consumption (median 0 mg vs 0–3 mg, p = 0.021) | No increase in complications | Extended analgesia time and improved block efficacy |
| Xiao R et al.[25] | TKA | IV loading 0.6 μg/kg + 0.2–0.4 μg/kg/h infusion + FNB | Equivalent to FNB + SNB in pain control | Similar rescue analgesia, propofol, and sufentanil use | No ↑ adverse events, ↑ extubation time (P < 0.001) | Safe alternative to SNB when contraindicated |
Table 1: Summary of clinical studies on dexmedetomidine use in anesthesia
Discussion
Current evidence suggests that dexmedetomidine may serve as an effective adjunct in perioperative pain management, with studies demonstrating potential benefits in analgesic efficacy and opioid-sparing effects. In a randomized controlled trial involving patients undergoing abdominal colectomy under general anesthesia, intraoperative administration of dexmedetomidine led to a significant reduction in postoperative morphine consumption and lower VAS pain scores during the first 24 hours, compared to those receiving a saline infusion. Likewise, in patients undergoing abdominal hysterectomy, intraoperative use of dexmedetomidine resulted in reduced morphine requirements and enhanced postoperative analgesia within the first postoperative day, further supporting its analgesic efficacy across a variety of abdominal surgical procedures.[27]
In orthopedic populations, particularly among patients undergoing total knee or hip arthroplasty, a meta-analysis demonstrated that dexmedetomidine significantly reduced pain scores at 24 hours postoperatively. Additionally, its use was associated with a lower incidence of postoperative delirium and decreased rates of nausea and vomiting, highlighting its multifaceted benefits in enhancing recovery. However, the analysis also noted a higher frequency of bradycardia in patients receiving dexmedetomidine, underscoring the need for careful cardiovascular monitoring during its use in these settings.[28]
Further evidence from regional anesthesia studies reinforces the utility of dexmedetomidine as an effective adjuvant. A meta-analysis of randomized controlled trials found that when dexmedetomidine was added to ropivacaine in brachial plexus blocks, it significantly accelerated the onset of anesthesia and prolonged the duration of both sensory and motor blockade. Notably, these benefits were achieved without a corresponding increase in the incidence of hypotension or bradycardia, supporting its safety and efficacy in peripheral nerve block applications.[29] In neurosurgical contexts, a pooled analysis of randomized controlled trials demonstrated that dexmedetomidine effectively reduced perioperative opioid consumption and decreased postoperative pain intensity in patients undergoing intracranial surgeries. These findings highlight its analgesic advantages even in complex procedures involving the central nervous system, where minimizing opioid use is particularly beneficial for neurological monitoring and recovery.[30]
A comprehensive review involving more than 2,400 critically ill patients compared dexmedetomidine to traditional sedatives and revealed a modest decrease in ICU length of stay, particularly when loading doses and high maintenance infusion rates were avoided. Crucially, the analysis found no significant increase in mortality or adverse cardiac events associated with dexmedetomidine use. However, the risk of bradycardia was notably higher with elevated dosing regimens, emphasizing the importance of dose optimization to balance efficacy with safety in critical care settings.
Perioperative use of dexmedetomidine has been consistently shown to decrease opioid requirements and enhance early postoperative pain management across diverse anesthesia settings. Its benefits extend beyond analgesia, contributing to faster recovery by reducing postoperative nausea and delirium and facilitating earlier mobilization. However, these advantages must be weighed against the dose-dependent risk of bradycardia and hypotension, particularly when administered as a loading bolus or at higher infusion rates. Careful dosing tailored to individual patient physiology, along with vigilant hemodynamic monitoring, is essential to ensure safety. While the existing literature predominantly addresses short-term outcomes, there remains considerable heterogeneity in surgical types and dosing protocols. Notably, data on long-term effects, especially in the management of chronic pain, are limited and warrant further investigation.
Conclusion
Current evidence indicates that dexmedetomidine may be an effective opioid-sparing agent with potential applications in both perioperative and regional anesthesia. Reported benefits include reduced pain scores, improved postoperative recovery profiles, and a lower incidence of opioid-related adverse effects. However, these findings are primarily derived from studies with relatively small sample sizes and limited patient diversity, which constrains the generalizability of the results. In addition, careful attention to dosing regimens and hemodynamic monitoring remains essential to mitigate known cardiovascular risks, particularly bradycardia and hypotension. Further large-scale, high-quality randomized controlled trials are warranted to establish standardized dosing protocols, evaluate long-term efficacy, and clarify the drug’s role across a broader range of surgical procedures and patient populations.
References
- Sykes AG, Oviedo P, Rooney AS, Gollin G. An assessment of dexmedetomidine as an opioid-sparing agent after neonatal open thoracic and abdominal operations. J Perinatol. 2022;42(3):307-312. doi:10.1038/s41372-021-01175-7
PubMed | Crossref | Google Scholar - Scott-Warren VL, Sebastian J. Dexmedetomidine: its use in intensive care medicine and anaesthesia. BJA Educ. 2016;16(7):242-246. doi:10.1093/bjaed/mkv047
Crossref | Google Scholar - Feng JF, Wang XX, Lu YY, Pang DG, Peng W, Mo JL. Effects of dexmedetomidine versus midazolam for premedication in paediatric anaesthesia with sevoflurane: A meta-analysis. J Int Med Res. 2017;45(3):912-923. doi:10.1177/0300060517704595
PubMed | Crossref | Google Scholar - Elvan EG, Oç B, Uzun S, Karabulut E, Coşkun F, Aypar U. Dexmedetomidine and postoperative shivering in patients undergoing elective abdominal hysterectomy. Eur J Anaesthesiol. 2008;25(5):357-364. doi:10.1017/S0265021507003110
PubMed | Crossref | Google Scholar - Kaye AD, Chernobylsky DJ, Thakur P, et al. Dexmedetomidine in Enhanced Recovery After Surgery (ERAS) Protocols for Postoperative Pain. Curr Pain Headache Rep. 2020;24(5):21. doi:10.1007/s11916-020-00853-z
PubMed | Crossref | Google Scholar - Viderman D, Aubakirova M, Nemerenova A, Salamat A, Abdildin YG. The Effects of Dexmedetomidine on Pain-Related Outcomes in Craniotomy: A Systematic Review and Meta-Analysis. World Neurosurg. 2024;190:e93-e108. doi:10.1016/j.wneu.2024.07.034
PubMed | Crossref | Google Scholar - Li B, Li Y, Tian S, et al. Anti-inflammatory Effects of Perioperative Dexmedetomidine Administered as an Adjunct to General Anesthesia: A Meta-analysis. Sci Rep. 2015;5:12342. doi:10.1038/srep12342
PubMed | Crossref | Google Scholar - Lee J, Hwang HW, Jeong JY, Kim YM, Park C, Kim JY. The Effect of Low-Dose Dexmedetomidine on Pain and Inflammation in Patients Undergoing Laparoscopic Hysterectomy. J Clin Med. 2022;11(10):2802. doi:10.3390/jcm11102802
PubMed | Crossref | Google Scholar - Ju T, Lee CC, Chen WC, Lin HT. USE OF DEXMEDETOMIDINE IN CRITICALLY ILL PATIENTS RECEIVING NONINVASIVE VENTILATION: A META-ANALYSIS OF RANDOMIZED CONTROLLED TRIALS. Chest. 2020;158(4):A577. doi:10.1016/j.chest.2020.08.545
PubMed | Crossref | Google Scholar - Barbaro S, Scapini E, Carone P, et al. Use of Dexmedetomidine as an Adjuvant in Spinal Anesthesia in Patients With Femoral Fracture Affected by Moderate Aortic Stenosis: A Case Series. Cureus. 2025;17(4):e83206. doi:10.7759/cureus.83206
PubMed | Crossref | Google Scholar - Zhao Y, He J, Yu N, Jia C, Wang S. Mechanisms of Dexmedetomidine in Neuropathic Pain. Front Neurosci. 2020;14:330. doi:10.3389/fnins.2020.00330
PubMed | Crossref | Google Scholar - Hu Y, Zhou H, Zhang H, et al. The neuroprotective effect of dexmedetomidine and its mechanism. Front Pharmacol. 2022;13:965661. doi:10.3389/fphar.2022.965661
PubMed | Crossref | Google Scholar - Mahmoud M, Mason KP. Dexmedetomidine: review, update, and future considerations of paediatric perioperative and periprocedural applications and limitations. Br J Anaesth. 2015;115(2):171-182. doi:10.1093/bja/aev226
PubMed | Crossref | Google Scholar - Ahmed WN, Khan AW. Use of dexmedetomidine for anesthesia and pain management: an updated review of literature. Anaesth Pain Intensive Care. 2022;26(5):702-709. doi:10.35975/apic.v26i5.2020
Crossref | Google Scholar - Reel B, Maani CV. Dexmedetomidine. In: StatPearls. Treasure Island (FL): StatPearls.
Dexmedetomidine - Hillman DR. Subspecialization … and Clinical Guidelines. Anesth Analg. 2018;127(4):815-816. doi:10.1213/ANE.0000000000003537
PubMed | Crossref | Google Scholar - Nallam SR, Kandala S, Kanipakam S, Bathini V, Chiruvella S, Sesham S. Cesarean Sections Under Spinal Anaesthesia: Comparison of Varying Doses of Dexmedetomidine Combined with 0.75% Hyperbaric Ropivacaine: A Double-Blind Randomized Trial. Turk J Anaesthesiol Reanim. 2024;52(4):134-141. doi:10.4274/TJAR.2024.241619
PubMed | Crossref | Google Scholar - Camporesi A, Mandelli A, Melloni E. Intrathecal nusinersen administration: Is anesthesia really needed?. Paediatr Anaesth. 2021;31(2):249-250. doi:10.1111/pan.14048
PubMed | Crossref | Google Scholar - Liang F, Liu M, Fu X, Zhou X, Chen P, Han F. Dexmedetomidine attenuates neuropathic pain in chronic constriction injury by suppressing NR2B, NF-κB, and iNOS activation. Saudi Pharm J. 2017;25(4):649-654. doi:10.1016/j.jsps.2017.04.039
PubMed | Crossref | Google Scholar - Dasta JF, Kane-Gill SL, Pencina M, et al. A cost-minimization analysis of dexmedetomidine compared with midazolam for long-term sedation in the intensive care unit. Crit Care Med. 2010;38(2):497-503. doi:10.1097/CCM.0b013e3181bc81c9
PubMed | Crossref | Google Scholar - Luo X, Hou HJ, Chen PS, et al. Addition of Dexmedetomidine to the Anesthesia Regimen Attenuates Pain and Improves Early Recovery After Esophageal Endoscopic Submucosal Dissection: A Randomized Controlled Trial. Drug Des Devel Ther. 2024;18:4551-4562. doi:10.2147/DDDT.S475749
PubMed | Crossref | Google Scholar - Azemati S, Keihani M, Sahmeddini MA, et al. Comparing the Sedative Effects of Intranasal Dexmedetomidine, Midazolam, and Ketamine in Outpatient Pediatric Surgeries: A Randomized Clinical Trial. Iran J Med Sci. 2024;49(7):421-429. doi:10.30476/ijms.2023.99122.3118
PubMed | Crossref | Google Scholar - Sangkum L, Termpornlert S, Tunprasit C, Rathanasutthajohn C, Komonhirun R, Dusitkasem S. Effect of low-dose dexmedetomidine to prolong spinal anesthesia in elderly patients: a prospective randomized controlled study. BMC Anesthesiol. 2024;24(1):427. doi:10.1186/s12871-024-02815-z
PubMed | Crossref | Google Scholar - Hamed MA, Fargaly OS, Abdelghaffar RA, Moussa MA, Algyar MF. The role of dexmedetomidine as an adjuvant for high-thoracic erector spinae plane block for analgesia in shoulder arthroscopy; a randomized controlled study. BMC Anesthesiol. 2023;23(1):53. doi:10.1186/s12871-023-02014-2
PubMed | Crossref | Google Scholar - Xiao R, Liu LF, Luo YR, et al. Dexmedetomidine Combined with Femoral Nerve Block Provides Effective Analgesia Similar to Femoral Nerve Combined with Sciatic Nerve Block in Patients Undergoing Total Knee Arthroplasty: A Randomized Controlled Study. Drug Des Devel Ther. 2022;16:155-164. doi:10.2147/DDDT.S334415
PubMed | Crossref | Google Scholar - Ge DJ, Qi B, Tang G, Li JY. Intraoperative Dexmedetomidine Promotes Postoperative Analgesia and Recovery in Patients after Abdominal Colectomy: A CONSORT-Prospective, Randomized, Controlled Clinical Trial. Medicine (Baltimore). 2015;94(43):e1727. doi:10.1097/MD.0000000000001727
PubMed | Crossref | Google Scholar - Ge DJ, Qi B, Tang G, Li JY. Intraoperative dexmedetomidine promotes postoperative analgesia and recovery in patients after abdominal hysterectomy: a double-blind, randomized clinical trial. Sci Rep. 2016;6:21514. doi:10.1038/srep21514
Crossref | Google Scholar - Yang Q, Ren Y, Feng B, Weng X. Pain relieving effect of dexmedetomidine in patients undergoing total knee or hip arthroplasty: A meta-analysis. Medicine (Baltimore). 2020;99(1):e18538. doi:10.1097/MD.0000000000018538
PubMed | Crossref | Google Scholar - Dai W, Tang M, He K. The effect and safety of dexmedetomidine added to ropivacaine in brachial plexus block: A meta-analysis of randomized controlled trials. Medicine (Baltimore). 2018;97(41):e12573. doi:10.1097/MD.0000000000012573
PubMed | Crossref | Google Scholar - Liu Y, Liang F, Liu X, Shao X, Jiang N, Gan X. Dexmedetomidine Reduces Perioperative Opioid Consumption and Postoperative Pain Intensity in Neurosurgery: A Meta-analysis. J Neurosurg Anesthesiol. 2018;30(2):146-155. doi:10.1097/ANA.0000000000000403
PubMed | Crossref | Google Scholar
Acknowledgments
Not reported
Funding
No funding
Author Information
Corresponding Author:
Samatha Ampeti, PhD
Independent Researcher, Department of Content
medtigo India Pvt Ltd, Pune, India
Email: ampetisamatha9@gmail.com
Co-Authors:
Patel Nirali Kirankumar, Mansi Srivastava, Raziya Begum Sheikh, Shubham Ravindra Sali, Sonam Shashikala B V
Independent Researcher, Department of Content
medtigo India Pvt Ltd, Pune, India
Authors Contributions
All authors contributed to the conceptualization, investigation, and data curation by acquiring and critically reviewing the selected articles. They were collectively involved in the writing – original draft preparation and writing – review & editing to refine the manuscript. Additionally, all authors participated in the supervision of the work, ensuring accuracy and completeness. The final manuscript was approved by all named authors for submission to the journal.
Ethical Approval
Not applicable
Conflict of Interest Statement
None
Guarantor
None
DOI
Cite this Article
Patel NK, Samatha A, Mansi S, Raziya BS, Shubham RS, Sonam SBV. Dexmedetomidine in Perioperative Pain Management: A Systematic Review of Analgesic Efficacy, Safety, and Clinical Applications. medtigo J Anesth Pain Med. 2025;1(2):e3067126. doi:10.63096/medtigo3067126 Crossref




