Author Affiliations
Abstract
Introduction: Gastric and duodenal ulcers, including those induced by endoscopic submucosal dissection (ESD), remain clinically important due to risks of delayed bleeding and impaired healing. Proton pump inhibitors (PPIs) like lansoprazole are a standard treatment. It is affected by the CYP2C19 polymorphisms. Vonoprazan, a potassium-competitive acid blocker (P-CAB), achieves rapid and potent acid suppression independent of CYP2C19 metabolism, offering potential advantages in post-ESD ulcer management.
Aim: The aim of this study is to compare the efficacy and safety of vonoprazan and PPIs in the gastric ulcer healing and prevention of post-ESD bleeding.
Methodology: A systematic review was conducted following PRISMA guidelines. PubMed and Google Scholar were searched (January 2015–June 2025) for randomized controlled trials (RCTs) and observational studies involving human subjects. Outcomes included ulcer healing rates, delayed bleeding, and safety events.
Result: Across multiple trials, vonoprazan and PPIs showed comparable overall efficacy in promoting ulcer healing, with >90% achieving scar-stage healing within 8 weeks. No significant difference was found in delayed bleeding or perforation rates. Subgroup analyses revealed potential benefits of vonoprazan in antithrombotic therapy patients, where bleeding risk was significantly lower than with PPIs. Preventive hemostasis during follow-up endoscopy was also less common with vonoprazan. The safety results were similar, with most adverse events being mild gastrointestinal symptoms.
Conclusion: Vonoprazan and PPIs are effective for post-ESD ulcer management. While both achieve comparable healing and safety, vonoprazan may be preferable in high-risk populations, particularly patients on antithrombotic therapy, due to its stable and potent acid suppression.
Keywords
Vonoprazan, Proton pump inhibitors, Potassium-Competitive acid blocker, Gastric ulcer, Endoscopic submucosal dissection.
Introduction
Gastric and duodenal ulcers continue to be a major clinical issue, primarily resulting from a disparity between gastric acid production and mucosal protective mechanisms.[1] Since the 1970s, acid suppression therapy has revolutionized the management of acid-related gastrointestinal disorders, with PPIs becoming the standard of care.[2] Despite their proven efficacy, PPIs are limited by pharmacogenetic variability, particularly CYP2C19 polymorphisms, which influence pharmacokinetics and pharmacodynamics, resulting in inconsistent clinical outcomes.[3,4] Lansoprazole, like other PPIs, irreversibly inhibits the gastric H⁺/K-ATPase pump in parietal cells but requires acid activation and is affected by hepatic metabolism.[5] In contrast, vonoprazan, a P-CAB, binds reversibly and directly to the proton pump without requiring activation and producing faster and more potent acid suppression with less influence from CYP2C19 metabolism.[6] This pharmacological difference is central to its clinical advantage in conditions requiring rapid and sustained acid control, like post-ESD ulcer management and severe gastroesophageal reflux disease.
ESD has been increasingly adopted since the early 2000s for resecting gastric neoplasms. It is linked with complications like hemorrhage, perforation, and stenosis.[7,8] Post-ESD hemorrhage, often defined as bleeding within four weeks, remains a serious complication. Current clinical guidelines recommend PPIs to suppress gastric acid and promote ulcer healing after ESD, with at least 2 weeks of therapy being advised.[9,10] However, pharmacogenetic limitations in PPIs have prompted evaluation of alternative therapies. Vonoprazan was introduced in 2015 and achieves rapid and sustained 24-hour acid suppression. Early clinical evidence suggests vonoprazan may be particularly effective in preventing delayed bleeding after ESD, especially in cases of larger ulcers, lesions with scarring, or ulcers located in the gastric antrum.[11,12] Comparative studies with lansoprazole report mixed findings. Some data suggest vonoprazan accelerates early ulcer healing, whereas PPIs, including lansoprazole, achieve comparable or even superior healing at later time points. A large multicenter study by Shiratori et al. discovered that while vonoprazan may be more effective in some subgroups, PPIs and vonoprazan were generally equally effective in stopping post-ESD bleeding.[13]
Recent systematic review evidence further clarifies this picture. In a meta-analysis of randomized and non-randomized trials, lansoprazole demonstrated superior ulcer healing and reduction compared with vonoprazan, while both drugs showed comparable efficacy in preventing post-ESD bleeding and perforation. These findings highlight vonoprazan’s therapeutic potential relative to traditional PPIs but also underscore the need for larger and more diverse studies.[14] Beyond healing rates, treatment choice has broader clinical and economic implications. Vonoprazan’s superior acid suppression may offer advantages in patients with high-risk bleeding ulcers, Helicobacter pylori-associated disease, or those resistant to PPIs.[15] However, PPIs like lansoprazole remain widely accessible, cost-effective, and supported by decades of safety data. Importantly, most ESD-induced ulcers heal to more than 90% within four weeks regardless of treatment, reinforcing the overall effectiveness of both therapies.[16] In erosive esophagitis, vonoprazan has demonstrated non-inferior healing rates compared to lansoprazole across multiple doses, with particularly high efficacy in severe cases and a comparable safety profile.[17]
Taken together, current evidence positions vonoprazan as a promising alternative to PPIs, particularly for patients with pharmacogenetic variability or severe acid-related disease.[18] However, the comparative efficacy of vonoprazan and lansoprazole in ulcer healing, bleeding prevention, and safety outcomes remains an area of active debate, necessitating further high-quality studies across broader clinical populations. The aim of this study is to systematically compare the efficacy and safety of vonoprazan, a potassium-competitive acid blocker, with proton pump inhibitors in promoting gastric ulcer healing and preventing delayed bleeding following ESD, with particular attention to outcomes in high-risk patient populations such as those receiving antithrombotic therapy.
Methodology
Following the preferred reporting items for systematic reviews and meta-analyses (PRISMA) guidelines, a systematic literature search was carried out. Relevant articles published between January 1, 2015, and June 30, 2025, were found using the PubMed and Google Scholar databases. The search strategy utilized combinations of the following keywords and Boolean operators: (“Vonoprazan” OR vonoprazan) AND (“Lansoprazole” OR lansoprazole) AND (“Proton Pump Inhibitors” OR PPI) AND (“Potassium-Competitive Acid Blockers” OR P-CAB) AND (“Gastrointestinal Ulcer” OR “gastrointestinal ulcer” OR “GI ulcer” OR “Stomach Ulcer” OR gastric ulcer OR “Duodenal Ulcer” OR duodenal ulcer OR (“Endoscopic Submucosal Dissection”).
A total of 764 records were initially retrieved from electronic databases. Following initial screening, 626 records were removed for reasons such as duplication, irrelevance, or failure to meet preliminary inclusion criteria. This left 138 records for detailed screening. Of these, 59 records were excluded after title and abstract review due to lack of relevance to the study objectives. The remaining 79 reports were sought for full-text retrieval, of which 47 could not be accessed. The 32 reports that were successfully retrieved underwent full-text assessment for eligibility. During this stage, 28 reports were excluded as they did not meet quality criteria, specifically achieving a scale for the assessment of narrative review articles (SANRA) score of less than 10. Ultimately, 4 studies met all inclusion criteria and were incorporated into the final review.
Inclusion criteria
- Original research articles (clinical trials, observational studies, randomized controlled trials).
- Studies conducted on human participants.
- Publications in English.
- Studies involving both male and female participants.
- Articles published between January 1, 2015, and June 30, 2025.
Exclusion criteria
- Non-research content (books, editorials, commentaries, letters, official documents, book chapters).
- Case reports, case series, and narrative reviews.
- Articles in languages other than English.
- Animal studies or in vitro research.
- Articles published outside the 2015–2025 timeframe.
- Studies lacking a results section.
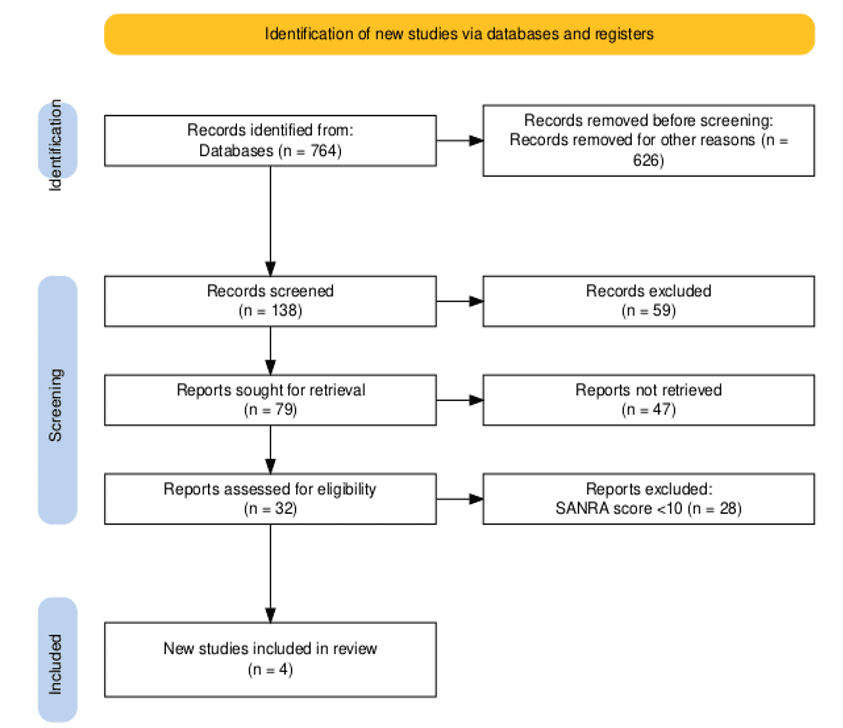
Figure 1: PRISMA diagram
Results
In a randomized controlled trial involving 414 patients undergoing ESD for gastric mucosal lesions, low-dose and high-dose PPI regimens demonstrated similar efficacy in preventing post-procedural complications. Following exclusions, 205 patients were given high-dose PPI, and 209 patients were given low-dose PPI. Age, sex, comorbid conditions, Helicobacter pylori infection, and mucosal atrophy were among the baseline characteristics that were similar across groups. The incidence of 7-day post-ESD bleeding was 6.2% in the low-dose group and 5.9% in the high-dose group with no significant difference (hazard ratio [HR] 0.94, 95% confidence interval [CI] 0.43 to 2.05, P = 0.869). Very few bleeding episodes required surgery or intensive care unit (ICU) treatment. The majority were treated endoscopically. No bleeding occurred beyond 7 days, and no procedure-related deaths were reported. At one month, endoscopic follow-up revealed comparable rates of active, healing, and scar-stage ulcers in both groups. Although hospital expenses were higher in the high-dose group, hospital stays were comparable. With the added advantage of lower treatment costs, these results imply that low-dose PPI therapy is not inferior to high-dose regimens in the management of post-ESD ulcers.[19]
Hamada et al. reported findings from a randomized controlled trial (RCT) and an observational study comparing vonoprazan with PPIs in the prevention of post-ESD bleeding. The RCT enrolled 139 patients (70 vonoprazan, 69 PPI), while the observational cohort included 408 vonoprazan and 870 PPI users. After propensity score matching, 392 patients were balanced in each group, with further stratification based on antithrombotic drug use. Baseline characteristics were well matched, with standardized differences <10%. In the overall population, post-ESD bleeding rates were comparable between groups (5.4% vonoprazan vs 5.6% PPI, risk difference −0.3%, 95% CI −3.4% to 2.9%). Pooled analysis of RCT and observational data suggested a treatment effect favoring vonoprazan (−4.3%, 95% CI −11.7% to 3.1%), though not statistically significant. Among patients not receiving antithrombotic therapy, bleeding rates remained low and similar across groups. However, in patients on antithrombotic agents, vonoprazan significantly reduced bleeding risk (9.3% vs 20.9% for PPIs), with a treatment effect of −11.6% (95% CI −22.2% to −1.1%). This benefit was confirmed in pooled analyses (−14.6%, 95% CI −22.0% to −7.2%). Subgroup analysis demonstrated particularly lower bleeding incidence in vonoprazan users receiving dual antiplatelet therapy or combined antiplatelet and anticoagulant therapy.[20]
Among 376 screened patients with gastric ulcers, 306 were randomized equally to tegoprazan 50 mg, tegoprazan 100 mg, or lansoprazole 30 mg. Twenty-eight patients (9.2%) discontinued due to withdrawal, adverse events, protocol violations, or loss to follow-up. Baseline characteristics were well balanced across groups, with most patients presenting with a single ulcer <10 mm and not taking nonsteroidal anti-inflammatory drugs (NSAIDs) or aspirin. In the per-protocol set, 8-week healing rates were 100% with tegoprazan 50 mg, 97.9% with tegoprazan 100 mg, and 100% with lansoprazole. At week 4, healing rates were 95.5%, 94.6%, and 92.9%, respectively. Both doses of tegoprazan were non-inferior to lansoprazole, and findings were consistent in the full analysis set, where cumulative 8-week healing exceeded 94% across all groups. Subgroup analyses showed similar outcomes in Helicobacter pylori-positive and -negative patients, with complete ulcer healing achieved by 8 weeks in all groups. Gastrointestinal symptoms improved comparably across treatments.
Approximately 304 patients had their safety assessed. Of the 66 patients who experienced 113 treatment-emergent adverse events (TEAEs), 9.8% experienced drug-related TEAEs (tegoprazan 50 mg), 13.7% experienced TEAEs (tegoprazan 100 mg), and 12% experienced TEAEs (lansoprazole 30 mg). Gastrointestinal conditions such as diarrhea, discomfort in the abdomen, and hypergastrinemia were the most frequent occurrences. Although there were serious TEAEs, they were not thought to be related to the treatment, and each group’s overall safety profile was similar. There were no discernible differences between tegoprazan and lansoprazole in serum gastrin levels, which rose slightly during treatment but fell back to baseline after stopping it.[21]
A total of 125 patients were enrolled, with 119 included in the final analysis (vonoprazan, n = 59; PPI, n = 60). The vonoprazan group’s lower body mass index (23 vs. 24, p = 0.04) was the only significant difference among the baseline demographic and clinical characteristics, which were otherwise balanced. About one-third of patients continued antithrombotic therapy after surgery, while most patients received monotherapy (88% vonoprazan vs. 77% PPI). Lesion size, resection time, and pathological findings were among the procedural characteristics that were similar across the groups. Delayed bleeding occurred in 13.6% of vonoprazan patients and 8.3% of PPI patients, with no significant difference (p = 0.39). Rates of perforation and immediate ulcer closure were also similar. However, preventive hemostasis during second-look endoscopy was more frequent in the PPI group (22% vs 5%, p = 0.01). At 8 weeks, scarring-stage ulcer healing was achieved in 76% of vonoprazan and 80% of PPI patients, indicating no difference in mucosal recovery.
Safety analyses revealed one cerebral infarction in the vonoprazan group, attributed to temporary rivaroxaban suspension, and one allergic reaction in the PPI group; no other severe drug-related adverse events were observed. Post-ESD symptoms measured by F-scale and visual analogue scale (VAS) showed no between-group differences, with moderate-to-severe pain reported in ~10% of patients on Day 1. Predictive analyses identified combination antithrombotic therapy as a strong risk factor for delayed bleeding, while pre-procedure antacid use was protective.[22]
Discussion
This study evaluated the comparative efficacy and safety of vonoprazan and PPIs in the management of post-ESD gastric ulcers. According to our research, there was no discernible difference in the incidence of delayed bleeding, perforation rates, or symptom-related outcomes between the two agents, and both showed high rates of ulcer healing at 8 weeks.[23] These results align with previous randomized and observational studies, which similarly report broadly equivalent efficacy between vonoprazan and PPIs in promoting post-ESD mucosal healing and preventing complications.[24]
Although overall bleeding rates were not significantly different, our data suggest a potential clinical advantage of vonoprazan in reducing the need for preventive hemostasis during follow-up endoscopy. This finding might be explained by vonoprazan’s pharmacological characteristics, which include quick, strong, and long-lasting acid suppression that is not dependent on CYP2C19 metabolism.[25,26] Prior studies have emphasized the potential benefit of vonoprazan in subgroups at high risk for post-ESD bleeding, particularly patients receiving antithrombotic therapy. Consistent with these findings, our subgroup analyses demonstrated that concomitant antithrombotic therapy, especially dual or combined regimens, was the strongest predictor of delayed bleeding, irrespective of the acid suppressant used.[27] Importantly, earlier reinitiation of antithrombotic therapy and lack of pre-procedural antacid use also emerged as significant risk factors, underscoring the multifactorial nature of post-ESD hemorrhage.
The complex role of vonoprazan in clinical practice is further highlighted by comparisons with earlier research. In patients taking antithrombotic medications, vonoprazan dramatically decreased the risk of bleeding, according to Hamada et al., while Shiratori et al. found that vonoprazan and PPIs were equally effective in larger populations. Similarly, meta-analytic data suggest that while PPIs may achieve slightly higher ulcer healing rates in the later stages, vonoprazan provides more consistent early acid suppression, which could be advantageous in settings requiring immediate gastric protection.[28] Our findings are concordant with this evidence, suggesting that vonoprazan may not universally outperform PPIs, but offers specific benefits in high-risk cohorts.
Clinically, vonoprazan and PPIs are both useful methods for managing post-ESD ulcers; by 8 weeks, more than 90% of ulcers have healed to the scar stage. PPIs continue to be the worldwide standard of care due to factors like cost, accessibility, and long-term safety data. Vonoprazan, on the other hand, might be better for patients who have CYP2C19 polymorphisms, have severe acid-related illnesses, or need ongoing antithrombotic treatment, where stable acid suppression is essential.[29,30]
There are a few limitations to consider. Despite the well-balanced baseline characteristics, generalizability may be limited by the single-country design and relatively small sample size. The statistical power to identify differences between treatment groups was diminished due to the rarity of bleeding events. Follow-up was restricted to 8 weeks, and longer-term outcomes such as ulcer recurrence, stricture formation, or sustained safety could not be assessed. To further elucidate the relative roles of vonoprazan and PPIs across various risk strata, future multicenter randomized controlled trials with larger, more diverse populations are required.
Conclusion
Vonoprazan and PPIs demonstrated comparable efficacy in promoting post-ESD ulcer healing and preventing delayed bleeding, with both agents achieving high scar-stage healing rates by 8 weeks. While no overall superiority was observed, vonoprazan showed potential advantages in reducing the need for preventive hemostasis and in patients receiving antithrombotic therapy, where stable acid suppression is critical. These results imply that both medications are still effective treatments, and the decision should be based on pharmacogenetic factors, patient risk factors, and medication availability.
References
- Malik TF, Gnanapandithan K, Singh K. Peptic Ulcer Disease. StatPearls Publishing; 2023.
Peptic Ulcer Disease - Scarpignato C, Gatta L, Zullo A, Blandizzi C; SIF-AIGO-FIMMG Group; Italian Society of Pharmacology, the Italian Association of Hospital Gastroenterologists, and the Italian Federation of General Practitioners. Effective and safe proton pump inhibitor therapy in acid-related diseases – A position paper addressing benefits and potential harms of acid suppression. BMC Med. 2016;14(1):179. doi:10.1186/s12916-016-0718-z
PubMed | Crossref | Google Scholar - El Rouby N, Lima JJ, Johnson JA. Proton pump inhibitors: from CYP2C19 pharmacogenetics to precision medicine. Expert Opin Drug Metab Toxicol. 2018;14(4):447-460. doi:10.1080/17425255.2018.1461835
PubMed | Crossref | Google Scholar - Eken E, Estores DS, Cicali EJ, Wiisanen KK, Johnson JA. A Pharmacogenetics-Based Approach to Managing Gastroesophageal Reflux Disease: Current Perspectives and Future Steps. Pharmgenomics Pers Med. 2023;16:645-664. doi:10.2147/PGPM.S371994
PubMed | Crossref | Google Scholar - Shin JM, Sachs G. Pharmacology of proton pump inhibitors. Curr Gastroenterol Rep. 2008;10(6):528-534. doi:10.1007/s11894-008-0098-4
PubMed | Crossref | Google Scholar - Song L, Zhang L. Tau Accumulation and Defective Autophagy: A Common Pathological Mechanism Underlying Repeat-Expansion-Induced Neurodegenerative Diseases? Neurosci Bull. 2020;36(12):1411-1413. doi:10.1007/s12264-020-00605-4
PubMed | Crossref | Google Scholar - Draganov PV, Aihara H, Karasik MS, et al. Endoscopic Submucosal Dissection in North America: A Large Prospective Multicenter Study. Gastroenterology. 2021;160(7):2317-2327.e2. doi:10.1053/j.gastro.2021.02.036
PubMed | Crossref | Google Scholar - Ma MX, Bourke MJ. Endoscopic submucosal dissection in the West: Current status and future directions. Dig Endosc. 2018;30(3):310-320. doi:10.1111/den.12960
PubMed | Crossref | Google Scholar - Kanaoka H, Iwatsubo T, Takeuchi T, et al. Is a proton-pump inhibitor necessary after endoscopic submucosal dissection for superficial esophageal neoplasms? A propensity score analysis. Therap Adv Gastroenterol. 2020;13:1756284820974908. doi:10.1177/1756284820974908
PubMed | Crossref | Google Scholar - Kataoka Y, Tsuji Y, Sakaguchi Y, et al. Bleeding after endoscopic submucosal dissection: Risk factors and preventive methods. World J Gastroenterol. 2016;22(26):5927-5935. doi:10.3748/wjg.v22.i26.5927
PubMed | Crossref | Google Scholar - Hamada K, Uedo N, Tonai Y, et al. Efficacy of vonoprazan in prevention of bleeding from endoscopic submucosal dissection-induced gastric ulcers: a prospective randomized phase II study. J Gastroenterol. 2019;54(2):122-130. doi:10.1007/s00535-018-1487-6
PubMed | Crossref | Google Scholar - Wong N, Reddy A, Patel A. Potassium-Competitive Acid Blockers: Present and Potential Utility in the Armamentarium for Acid Peptic Disorders. Gastroenterol Hepatol (N Y). 2022;18(12):693-700.
Potassium-Competitive Acid Blockers: Present and Potential Utility in the Armamentarium for Acid Peptic Disorders - Shiratori Y, Niikura R, Ishii N, Ikeya T, Honda T, Hasatani K, Fukuda K. Vonoprazan versus proton pump inhibitors for postendoscopic submucosal dissection bleeding in the stomach: a multicenter population-based comparative study. Gastrointest Endosc. 2022;95(1):72-79. doi:10.1016/j.gie.2021.06.032
Crossref | Google Scholar - Ameer Hamza M-ul-H, Nehala N, Jawad Zaidi SM. Vonoprazan vs Lansoprazole in Gastrointestinal Tract Ulcers: A Systematic Review and Meta-Analysis. medtigo J Med.2024;2(3):e3062239. doi:10.63096/medtigo3062239
Crossref - Ban H, Inatomi O, Murata M, et al. Vonoprazan vs lansoprazole for the treatment of artificial gastric ulcer after endoscopic submucosal dissection: a prospective randomized comparative study. J Clin Biochem Nutr. 2021;68(3):259-263. doi:10.3164/jcbn.20-143
PubMed | Crossref | Google Scholar - Takahashi K, Sato Y, Kohisa J, et al. Vonoprazan 20 mg vslansoprazole 30 mg for endoscopic submucosal dissection-induced gastric ulcers. World J Gastrointest Endosc. 2016;8(19):716-722. doi:10.4253/wjge.v8.i19.716
PubMed | Crossref | Google Scholar - Ashida K, Sakurai Y, Nishimura A, et al. Randomised clinical trial: a dose-ranging study of vonoprazan, a novel potassium-competitive acid blocker, vs. lansoprazole for the treatment of erosive oesophagitis. Aliment Pharmacol Ther. 2015;42(6):685-695. doi:10.1111/apt.13331
PubMed | Crossref | Google Scholar - Padwale V, Kirnake V, Daswani R, Kodmalwar A, Gupta A. A Comprehensive Review on the Efficacy and Safety of Vonoprazan in the Management of Gastric Acid-Related Diseases. Cureus. 2024;16(7):e64777. doi:10.7759/cureus.64777
PubMed | Crossref | Google Scholar - Yang L, Qi J, Chen W, et al. Low-dose PPI to prevent bleeding after ESD: A multicenter randomized controlled study. Biomed Pharmacother. 2021;136:111251. doi:10.1016/j.biopha.2021.111251
PubMed | Crossref | Google Scholar - Hidaka Y, Imai T, Inaba T, Kagawa T, Omae K, Tanaka S. Efficacy of vonoprazan against bleeding from endoscopic submucosal dissection-induced gastric ulcers under antithrombotic medication: A cross-design synthesis of randomized and observational studies. PLoS One. 2021;16(12):e0261703. doi:10.1371/journal.pone.0261703
PubMed | Crossref | Google Scholar - Cho YK, Choi MG, Choi SC, et al. Randomised clinical trial: tegoprazan, a novel potassium-competitive acid blocker, or lansoprazole in the treatment of gastric ulcer. Aliment Pharmacol Ther. 2020;52(5):789-797. doi:10.1111/apt.15865
PubMed | Crossref | Google Scholar - Shibata H, Fujiyoshi T, Kawata N, et al. Vonoprazan or Proton Pump Inhibitor for Gastric Endoscopic Submucosal Dissection in Patients Taking Antithrombotic Agents. J Gastroenterol Hepatol. 2025;40(8):1890-1898. doi:10.1111/jgh.17027
PubMed | Crossref | Google Scholar - Tarasconi A, Coccolini F, Biffl WL, et al. Perforated and bleeding peptic ulcer: WSES guidelines. World J Emerg Surg. 2020;15:3. doi:10.1186/s13017-019-0283-9
PubMed | Crossref | Google Scholar - Martin, Zhou Y, Meng CX, Takagi T, Tian YS. Vonoprazan vs proton pump inhibitors in treating post-endoscopic submucosal dissection ulcers and preventing bleeding: A meta-analysis of randomized controlled trials and observational studies. Medicine (Baltimore). 2020;99(9):e19357. doi:10.1097/MD.0000000000019357
PubMed | Crossref | Google Scholar - Maselli R, Da Rio L, Manno M, et al. Efficacy of novel endoscopic hemostatic agent for bleeding control and prevention: Results from a prospective, multicenter national registry. Endosc Int Open. 2024;12(10):E1220-1229. doi:10.1055/a-2406-7492
PubMed | Crossref | Google Scholar - Pattarapuntakul T, Wong T, Wetwittayakhlang P, et al. Efficacy of Vonoprazan vs. Intravenous Proton Pump Inhibitor in Prevention of Re-Bleeding of High-Risk Peptic Ulcers: A Randomized Controlled Pilot Study. J Clin Med. 2024;13(12):3606. doi:10.3390/jcm13123606
PubMed | Crossref | Google Scholar - Gotoda T, Hori K, Iwamuro M, et al. Evaluation of the bleeding risk with various antithrombotic therapies after gastric endoscopic submucosal dissection. Endosc Int Open. 2017;5(7):E653-662. doi:10.1055/s-0043-110050
PubMed | Crossref | Google Scholar - Oshima T, Miwa H. Potent Potassium-competitive Acid Blockers: A New Era for the Treatment of Acid-related Diseases. J Neurogastroenterol Motil. 2018;24(3):334-344. doi:10.5056/jnm18029
PubMed | Crossref | Google Scholar - Chen L, Jiang D, Hu D, Cui X. Comparison of vonoprazan and proton pump inhibitors for the treatment of gastric endoscopic submucosal dissection-induced ulcer: an updated systematic review and meta-analysis. BMC Gastroenterol. 2024;24(1):110. doi:10.1186/s12876-024-03198-8
PubMed | Crossref | Google Scholar - Setia A, Challa RR, Vallamkonda B, Vaishali, Viswanadh MK, Muthu MS. Clinical Implications of Proton Pump Inhibitors and Vonoprazan Micro/Nano Drug Delivery Systems for Gastric Acid-Related Disorders and Imaging. Nanotheranostics. 2024;8(4):535-560. doi:10.7150/ntno.100727
PubMed | Crossref | Google Scholar
Acknowledgments
Not reported
Funding
No funding
Author Information
Corresponding Author:
Samatha Ampeti, PhD
Independent Researcher, Department of Content
medtigo India Pvt Ltd, Pune, India
Email: ampetisamatha9@gmail.com
Co-Authors:
Patel Nirali Kirankumar, Mansi Srivastava, Raziya Begum Sheikh, Shubham Ravindra Sali, Independent Researcher, Department of Content
medtigo India Pvt Ltd, Pune, India
Authors Contributions
All authors contributed to the conceptualization, investigation, and data curation by acquiring and critically reviewing the selected articles. They were collectively involved in the writing – original draft preparation and writing – review & editing to refine the manuscript. Additionally, all authors participated in the supervision of the work, ensuring accuracy and completeness. The final manuscript was approved by all named authors for submission to the journal.
Ethical Approval
Not applicable
Conflict of Interest Statement
None
Guarantor
None
DOI
Cite this Article
Patel NK, Samatha A, Mansi S,Raziya BS, Shubham RS. Comparative Efficacy of Vonoprazan and Proton Pump Inhibitors in Gastrointestinal Ulcer Healing and Post-ESD Bleeding Prevention. medtigo J Emerg Med. 2025;2(3):e30922311. doi:10.63096/medtigo30922311 Crossref




